
This wavefunction is the stationary state, and the scaling factor is the energy. Eventually you will get a wavefunction for which the left-hand side looks very close to a scaled version of $\psi$ (or equivalently, for which the left-hand side after normalization looks very close to $\psi$). If you're making a guess for $\psi$, then you can evaluate the left-hand side of the equation, determine how similar it is to $\psi$, adjust your guess in the direction that your result indicates, and evaluate again. of the hydrogen atom, following Schrdingers original solution. Suppose the three dimensions of our box are the same. In his first paper, he solved the Schrdinger equation using the Laplace method.
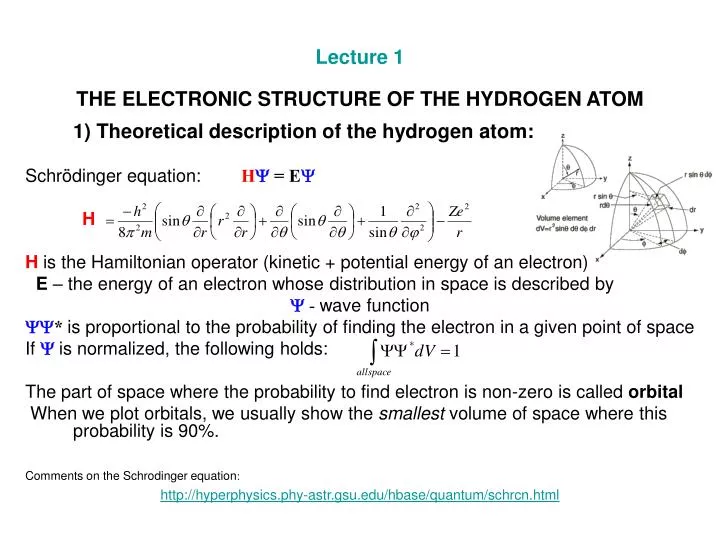

In the case of the hydrogen atom, the boundary conditions are much different, but they also lead to three spatial quantum numbers. Consider following Schrodinger wave equation for an orbital of hydrogen atom. Repeat this process enough times, and you may zero in on a value of $E$ that produces a wavefunction $\psi$ that fits the necessary conditions. The solution to the Schrodinger equation in three dimensions led to three quantum numbers. But of course, the Hydrogen atom does not live in one dimension. If the wavefunction is not "sensible," then, based on the particular way that $\psi$ is not "sensible," you make another guess for $E$ that is more likely to make the wavefunction "sensible" (for example, in the hydrogen atom, if the wavefunction grows indefinitely with distance, we might choose a lower value of energy in the next iteration). The Hydrogen Calculation using Schrdingers equation.

If you're making a guess for $E$, then you proceed by solving the above PDE for $\psi$ and checking if the wavefunction is "sensible" (the conditions for "sensible" depend on the situation for the hydrogen atom, we might include "vanishes at infinity" in our definition of "sensible," because hydrogen atoms have finite spatial extent). In the most basic quantum mechanical model of hydrogen, the proton is taken to be a fixed source of an electric potential and the Schrdinger equation for. There are generally two ways to start solving this - either start with a guess for $E$ and solve for $\psi$, or start with a guess for $\psi$ and solve for $E$. The hydrogen atom, in the rest frame of the proton, has as its only nontrivial component a single electron, with wavefunction $\psi(\vec\psi=E\psi$$ Hydrogen Atom Waves and Light Paradoxes in Classical Physics Planck, Einstein, and Bohr Waves, Particles, and the Schrödinger equation The Hydrogen Atom.


 0 kommentar(er)
0 kommentar(er)
